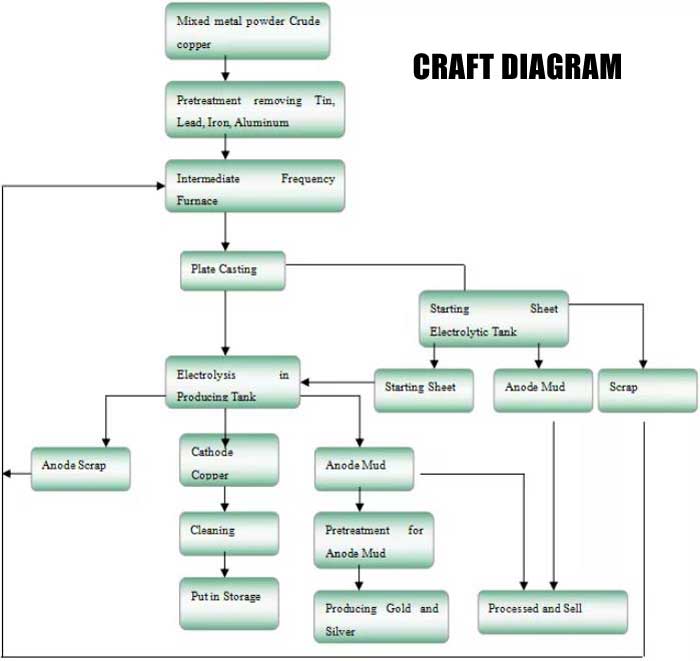
The blister copper (containing 99% copper) is pre-made into a thick plate as the anode, the pure copper is made into a thin sheet as the cathode, and the mixed solution of sulfuric acid and copper sulfate is used as the electrolyte. After electrification, copper dissolves from the anode into copper ions (Cu) and moves to the cathode. After reaching the cathode, electrons are obtained and pure copper (also known as electrolytic copper) is precipitated at the cathode. Impurities in blister copper, such as iron and zinc, which are more active than copper, will dissolve together with copper as ions (Zn and Fe).
Since these ions are less likely to be precipitated than copper ions, the precipitation of these ions on the cathode can be avoided as long as the potential difference is properly adjusted during electrolysis. Impurities less reactive than copper, such as gold and silver, are deposited on the bottom of the cell. The copper plates produced in this way, called "electrolytic copper", are of extremely high quality and can be used to make electrical products.
By further processing the electrolytic copper, it can be made into extremely fine electrolytic copper powder.
The principle of copper electrolytic refining is as follows:
Anodic reaction: Cu — 2e- = Cu2+
Me — 2e-= Me2+
H2O — 2e- = 2H+ + 1/2O2
SO4 2- — 2e- = SO3 + 1/2O2
In the formula, Me represents Fe, Ni, Pb, As, Sb and other metals that are more electronegative than Cu, which dissolve into solution from the anode. The reaction of H2O and SO4 2- losing electrons does not occur under normal conditions because its potential is more positive than that of copper. The potential of the precious metal is corrected, and it does not dissolve and enters the anode slime.
Cathodic reaction: Cu2+ + 2e = Cu
2H+ + 2e- = H2
Me2+ + 2e- = Me
In these reactions, metal ions with a positive standard potential and a higher concentration of copper may be reduced at the cathode, but they are not dissolved at the anode, so only the reduction of copper ions is the main reaction of the cathode reaction.
SUNY GROUP's copper electrolysis treatment and reuse equipment mainly purifies crude copper according to the principle of copper electrolysis. Metals, rare earth metals and precious metals, often passivate the anode in conventional electrolysis, which makes the electrolysis unable to proceed normally. It is recommended to use a pulsed automatic pole-changing electrolysis system, which not only effectively solves the problem of anode passivation, but also helps to loosen and fall off the anode slime. The time adjustment is arbitrarily set by the cycle time relay on the panel of the electrolysis control cabinet.
The copper electrolytic treatment and reuse equipment is composed of multiple IGBT automatic power control modules and belongs to digital pulse power equipment. When casting the anode plate, a semi-automatic vertical mold is used. In order to shorten the electrolysis cycle, the thickness of the anode is generally controlled at 10 mm to 30 mm. Anode slime, clean the anode slime, conduct reduction smelting and oxidation smelting in intermediate frequency furnace and blowing furnace, smelted precious metal alloy blocks are crushed by water, and then go through precious metal liquid making to extract, separate and refine various precious metals. In the production workshop, a tail gas absorption device with ozone must be set up in all places where tail gas is generated, and a tail liquid diversion device must be set up in all places where tail liquid is generated, and the tail liquid is diverted to the wastewater treatment workshop and discharged or reused after reaching the standard.
Thank you for your interest in suny group. If you want to learn more about our E-waste recycling plant, copper wire recycling machine and other machines, Contact us now to find out what we can do for you next project!E-mail:sunymachine@gmail.com | Whatsapp:+8613674945231